Abstract
The species of Hypostomus from the Parnaíba River basin were reviewed through molecular and morphological analysis. Five species were found in the basin, including a new species herein described. The distribution of H. pusarum was expanded to this basin, and a closely related species was recorded (H. aff. pusarum), also the presence of H. johnii and H. vaillanti was confirmed. The new species is distinguished from most congeners by its large number of premaxillary and dentary teeth, a wide dental angle of 115° to 135°, presence of a rounded dark spots on a lighter background and anteromedial region of the abdomen depleted of plaques (vs. anteromedial region of the abdomen covered by platelets and odontodes in H. johnii, H. pusarum, H. aff. pusarum and H. vaillanti). Furthermore, an identification key of the species from the Maranhão-Piauí ecoregion and maps with the geographic distribution of these species are presented. The species of Hypostomus in the Parnaíba River basin have different geographic distributions, suggesting different niches or geographical barriers, providing an opportunity for ecological and evolutionary studies.
Keywords:
Cryptic diversity; DNA Barcode; Identification key; Integrative taxonomy; Maranhão-Piauí ecoregion
Resumo
As espécies de Hypostomus da bacia do rio Parnaíba foram revisadas por meio de análises moleculares e morfológicas. Cinco espécies foram encontradas na bacia, incluindo uma nova espécie aqui descrita. A distribuição de H. pusarum foi expandida para esta bacia, uma espécie intimamente relacionada foi registrada (H. aff. pusarum), e a presença de H. johnii e H. vaillanti foi confirmada. A nova espécie se distingue da maioria das congêneres por seu grande número de dentes nos pré-maxilares e dentários, um amplo ângulo do dentário de 115° a 135°, presença de manchas escuras arredondadas em um fundo mais claro e região anteromedial do abdômen sem placas (vs. região anteromedial do abdômen coberta por placas e odontódios em H. johnii, H. pusarum, H. aff. pusarum e H. vaillanti). Além disso, é apresentada uma chave de identificação das espécies da ecorregião Maranhão-Piauí e mapas com a distribuição geográfica dessas espécies. As espécies de Hypostomus na bacia do rio Parnaíba apresentam diferentes distribuições geográficas, sugerindo diferentes nichos ou barreiras geográficas, proporcionando oportunidade para estudos ecológicos e evolutivos.
Palavras-chave:
Chave de identificação; Diversidade críptica; DNA Barcode; Ecorregião Maranhão-Piauí; Taxonomia integrativa
INTRODUCTION
The genus Hypostomus Lacépède, 1803, with approximately 142 valid species, is the most speciose within the family Loricariidae (Lujan et al., 2015Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogeneti Evol. 2015; 82:269–88. https://doi.org/10.1016/j.ympev.2014.08.020
https://doi.org/10.1016/j.ympev.2014.08....
; De Queiroz et al., 2020De Queiroz LJ, Cardoso Y, Jacot-des-Combes C, Bahechar IA, Lucena CA, Py-Daniel LR, Torrente-Vilara G. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus.Mol Phylogenet Evol. 2020; 145:106711. https://doi.org/10.1016/j.ympev.2019.106711
https://doi.org/10.1016/j.ympev.2019.106...
; Fricke et al., 2021Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2021 http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.as
http://researcharchive.calacademy.org/re...
; Penido et al., 2021Penido IS, Pessali TC, Zawadzki CH. When destruction comes first: Two new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from a deeply-impacted river in the Rio São Francisco basin in Brazil. J Fish Biol. 2021; 98(5):1371–84. https://doi.org/10.1111/jfb.14674
https://doi.org/10.1111/jfb.14674...
). Over the last years, this group has been the focus of many systematic studies due to its taxonomic complexity (Montoya-Burgos, 2003Montoya-Burgos JI. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol. 2003; 12(7):1855–67. https://doi.org/10.1046/j.1365-294X.2003.01857.x
https://doi.org/10.1046/j.1365-294X.2003...
; Cardoso et al., 2012Cardoso YP, Almiron A, Casciotta J, Aichino D, Lizarralde MS, Montoya-Burgos JI. Origin of species diversity in the catfish genus Hypostomus (Siluriformes: Loricariidae) inhabiting the Paraná River basin, with the description of a new species. Zootaxa. 2012; 3453(1):69–83. https://doi.org/10.11646/zootaxa.3453.1.5
https://doi.org/10.11646/zootaxa.3453.1....
; Silva et al., 2016Silva GSC, Roxo FF, Lujan NK, Tagliacollo VA, Zawadzki CH, Oliveira C. Transcontinental dispersal, ecological opportunity and origins of an adaptive radiation in the Neotropical catfish genus Hypostomus (Siluriformes: Loricariidae). Mol Ecol. 2016; 25(7):1511–29. https://doi.org/10.1111/mec.13583
https://doi.org/10.1111/mec.13583...
; De Oliveira Brandão et al., 2018De Oliveira Brandão K, Rocha-Reis DA, Garcia C, Pazza R, de Almeida-Toledo LF, Kavalco KF. Studies in two allopatric populations of Hypostomus affinis (Steindachner, 1877): the role of mapping the ribosomal genes to understand the chromosome evolution of the group. Comp Cytogenet. 2018; 12(1):1–12. https://doi.org/10.3897/CompCytogen.v12i1.22052
https://doi.org/10.3897/CompCytogen.v12i...
; Dias, Zawadzki, 2018Dias AC, Zawadzki CH. Identification key and pictures of the Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) from the rio Ivaí, upper rio Paraná basin.Check List. 2018; 14(2):393–414. https://doi.org/10.15560/14.2.393
https://doi.org/10.15560/14.2.393...
; Cardoso et al., 2019Cardoso YP, Brancolini F, Protogino L, Paracampo A, Bogan S, Posadas P, Montoya-Burgos JI. An integrated approach clarifies the cryptic diversity in Hypostomus Lacépède 1803 from the Lower La Plata Basin. An Acad Bras Cienc. 2019; 91(2). https://doi.org/10.1590/0001-3765201920180131
https://doi.org/10.1590/0001-37652019201...
; Anjos et al., 2020Anjos MS, Bitencourt JA, Nunes LA, Sarmento-Soares LM, Carvalho DC, Armbruster JW, Affonso PR. Species delimitation based on integrative approach suggests reallocation of genus in Hypostomini catfish (Siluriformes, Loricariidae). Hydrobiologia. 2020; 847(2):563–78. https://link.springer.com/article/10.1007/s10750-019-04121-z
https://link.springer.com/article/10.100...
; De Queiroz et al., 2020De Queiroz LJ, Cardoso Y, Jacot-des-Combes C, Bahechar IA, Lucena CA, Py-Daniel LR, Torrente-Vilara G. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus.Mol Phylogenet Evol. 2020; 145:106711. https://doi.org/10.1016/j.ympev.2019.106711
https://doi.org/10.1016/j.ympev.2019.106...
). Species of Hypostomus are morphologically characterized by having a dorsoventrally flattened body covered by bony plates, and for inhabiting the most diverse kind of habitats, from fast-flowing rivers with rocky bottom to lentic and turbid water and muddy substrate (Weber, 2003Weber C. The Hypostominae. In: Reis RE, Kullander SO, Ferraris Jr. CJ, editors. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs. 2003; p.351–72. ; Casatti et al., 2005Casatti L, Rocha FC, Pereira DC. Habitat use by two species of Hypostomus (Pisces, Loricariidae) in southeastern Brazilian streams. Biota Neotrop. 2005; 5(2):157–65.; Lujan et al., 2015Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogeneti Evol. 2015; 82:269–88. https://doi.org/10.1016/j.ympev.2014.08.020
https://doi.org/10.1016/j.ympev.2014.08....
; Sá-Oliveira, Isaac, 2015Sá-Oliveira JC, Isaac VJ. Diet breadth and niche overlap between Hypostomus plecostomus (Linnaeus, 1758) and Hypostomus emarginatus (Valenciennes, 1840) (Siluriformes) from the coaracy nunes hydroelectric reservoir in Ferreira Gomes, Amapá-Brazil. Biota Amaz. 2015; 3(2). http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v3n2p116-125
http://dx.doi.org/10.18561/2179-5746/bio...
).
The great number of described species coupled to the phenotypic plasticity and ontogenetic variation are currently a challenge for the identification of Hypostomus’ species. Therefore, the use of integrative taxonomy, combining morphological and molecular tools, can be crucial for elucidating doubts regarding species’ identity, their geographical distribution, and their phylogenetic relationships (Zawadzki et al., 2012Zawadzki CH, Birindelli JLO, Lima FCT. A new armored catfish species of the genus Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the upper rio Xingu basin, Brazil. Neotrop Ichthyol. 2012; 10(2):245–53. https://doi.org/10.1590/S1679-62252012000200003
https://doi.org/10.1590/S1679-6225201200...
; Zanata, Pitanga, 2016Zanata AM, Pitanga BR. A new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from rio Itapicuru basin, Bahia State, Brazil. Zootaxa. 2016; 4137(2):223–32. https://doi.org/10.11646/zootaxa.4137.2.4
https://doi.org/10.11646/zootaxa.4137.2....
; Anjos et al., 2020Anjos MS, Bitencourt JA, Nunes LA, Sarmento-Soares LM, Carvalho DC, Armbruster JW, Affonso PR. Species delimitation based on integrative approach suggests reallocation of genus in Hypostomini catfish (Siluriformes, Loricariidae). Hydrobiologia. 2020; 847(2):563–78. https://link.springer.com/article/10.1007/s10750-019-04121-z
https://link.springer.com/article/10.100...
; Cardoso et al., 2019Cardoso YP, Brancolini F, Protogino L, Paracampo A, Bogan S, Posadas P, Montoya-Burgos JI. An integrated approach clarifies the cryptic diversity in Hypostomus Lacépède 1803 from the Lower La Plata Basin. An Acad Bras Cienc. 2019; 91(2). https://doi.org/10.1590/0001-3765201920180131
https://doi.org/10.1590/0001-37652019201...
).
In the hydrographic basins of Northeastern Brazil’s ecoregions, there are currently 23 described species of Hypostomus, of which 12 occur in São Francisco ecoregion (SFRE), seven in Mid-Northeastern Caatinga (MNCE), seven in Northeastern Atlantic Forest (NAFE), and two in Maranhão-Piauí (MAPE) (Reis et al., 2003Reis RE, Kullander SO, Ferraris Jr, C. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs. 2003.; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
; Barbosa et al., 2017Barbosa JM, Soares EC, Cintra IHA, Hermann M, Araújo ARR. Perfil da ictiofauna da bacia do rio São Francisco/Profile of the fish fauna of the São Francisco river basin. Acta Fish Aquat Res. 2017; 5(1):70–90. https://seer.ufs.br/index.php/ActaFish/article/view/5862
https://seer.ufs.br/index.php/ActaFish/a...
; Lima et al., 2017Lima SMQ, Ramos TPA, Silva MJ, Rosa RS. Diversity, distribution, and conservation of the caatinga fishes: advances and challenges. In: Silva JMC, Leal IR, Tabarelli M., editors. Caatinga. Springer; 2017. p.97–131.; Penido et al., 2021Penido IS, Pessali TC, Zawadzki CH. When destruction comes first: Two new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from a deeply-impacted river in the Rio São Francisco basin in Brazil. J Fish Biol. 2021; 98(5):1371–84. https://doi.org/10.1111/jfb.14674
https://doi.org/10.1111/jfb.14674...
). MAPE’s drainages are in the domain of Caatinga and Cerrado biomes, and the Parnaíba River basin is its main system, and the second largest hydrographic basin in Northeastern Brazil (Rosa et al., 2003Rosa RS, Menezes NA, Britski HA, Costa WJE, Groth F. Diversidade, padrões de distribuição e conservação dos peixes da caatinga; In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e Conservação da Caatinga. Recife: Editora Universitária da Universidade Federal de Pernambuco. 2003; p.135–80. ; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
). In the Parnaíba River basin two nominal species are recognized, Hypostomus johnii (Steindachner, 1877) and Hypostomus vaillanti (Steindachner, 1877), and four putative new species according to Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, 2017Ramos TPA, Zawadzki CH, Ramos RTDC, Britski HA. Redescription of Hypostomus johnii, a senior synonym of Hypostomus eptingi (Siluriformes: Loricariidae), Northeastern Brazil. Neotrop Ichthyol. 2017; 15(2):e160064. https://doi.org/10.1590/1982-0224-20160064
https://doi.org/10.1590/1982-0224-201600...
), and Lima et al., (2017)Lima SMQ, Ramos TPA, Silva MJ, Rosa RS. Diversity, distribution, and conservation of the caatinga fishes: advances and challenges. In: Silva JMC, Leal IR, Tabarelli M., editors. Caatinga. Springer; 2017. p.97–131., however without giving neither their diagnostic characters nor their respective geographic distributions.
The Parnaíba River has a perennial regime, but some tributaries are temporary, and it can be considered a transition zone between the semiarid Caatinga in the east of the basin, and the Cerrado in the west (Rosa et al., 2003Rosa RS, Menezes NA, Britski HA, Costa WJE, Groth F. Diversidade, padrões de distribuição e conservação dos peixes da caatinga; In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e Conservação da Caatinga. Recife: Editora Universitária da Universidade Federal de Pernambuco. 2003; p.135–80. ). Even though it is considered the largest hydrographic basin fully inserted in Northeast of Brazil, the Parnaíba River lacks studies of its ichthyofauna. Authors such as Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, Silva et al., (2015)Silva MJ, Costa BG, Ramos TPA, Auricchio P, Lima SMQ. Ichthyofauna of the Gurgueia river, Parnaíba river basin, northeastern Brazil. Check List. 2015; 11(5):1–8. https://doi.org/10.15560/11.5.1765
https://doi.org/10.15560/11.5.1765...
and Melo et al., (2016)Melo FAG, Buckup PA, Ramos TPA, do Nascimento Souza AK, Silva CMA, Costa TC, Torres AR. Fish fauna of the lower course of the Parnaíba River, northeastern Brazil. Bol Mus Biol Mello Leitão. 2016; (4):363–400. contributed recently with surveys of fish species in this basin, however, according to Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, Parnaíba has a high endemism (38.9%), with about 27 taxa not yet described.
Considering that taxonomic studies are paramount to evaluate the conservation status of a species (Cardoso et al., 2019Cardoso YP, Brancolini F, Protogino L, Paracampo A, Bogan S, Posadas P, Montoya-Burgos JI. An integrated approach clarifies the cryptic diversity in Hypostomus Lacépède 1803 from the Lower La Plata Basin. An Acad Bras Cienc. 2019; 91(2). https://doi.org/10.1590/0001-3765201920180131
https://doi.org/10.1590/0001-37652019201...
), we revised the species of Hypostomus from the Parnaíba River basin through integrative taxonomy (morphological and molecular data), delimiting their geographical distribution, and preliminarily inferring their phylogenetic relationships. Additionally, we describe a new endemic species from the basin and propose an identification key of the genus from Maranhão-Piauí ecoregion.
MATERIAL AND METHODS
Study area. The Maranhão-Piauí ecoregion comprises areas of Amazon Forest to the west and semiarid Caatinga to the east, but its largest portion is in the Cerrado between both. This ecoregion encompasses small coastal basins in the east and the major Parnaíba River basin in the states of Maranhão, Piauí, and Ceará (Rosa et al., 2003Rosa RS, Menezes NA, Britski HA, Costa WJE, Groth F. Diversidade, padrões de distribuição e conservação dos peixes da caatinga; In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e Conservação da Caatinga. Recife: Editora Universitária da Universidade Federal de Pernambuco. 2003; p.135–80. ). This river is perennial, with a few intermittent tributaries in the Caatinga (Rosa et al., 2003Rosa RS, Menezes NA, Britski HA, Costa WJE, Groth F. Diversidade, padrões de distribuição e conservação dos peixes da caatinga; In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e Conservação da Caatinga. Recife: Editora Universitária da Universidade Federal de Pernambuco. 2003; p.135–80. ; Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
).
Data sampling. Specimens were collected using several types of fishing gear (trawl, cast nets, gill nets and sieves), and they were anesthetized in the field with eugenol following Lucena et al., (2013)Lucena CAS, Calegari BB, Pereira EHL, Dallegrave E. O uso de óleo de cravo na eutanásia de peixes. Bol Soc Bras Ictiologia. 2013; 105:20–24., and, posteriorly fixed in formalin 10%. After a few days, specimens were transferred to ethanol 70% (Malabarba, Reis, 1987Malabarba LR, Reis RE. Manual de técnicas para a preparação de coleções zoológicas.Soc Bras Zool. 1987; 36:1–14.) and deposited in the ichthyological collections of Universidade Federal da Paraíba, João Pessoa (UFPB), Universidade Federal do Rio Grande do Norte, Natal (UFRN), Museu de Zoologia da Universidade de São Paulo, São Paulo (MZUSP), and Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura, Universidade Estadual de Maringá, Maringá (NUP). The individuals that were used in molecular analyses had a portion of their pelvic fin or muscular tissue removed still in the field and fixed in ethanol P.A. (99%). Afterwards, we tagged them individually and tissues were deposited in the tissue collection of the Universidade Federal do Rio Grande do Norte (UFRN). Other abbreviations: Academy of Natural Sciences of Drexel University, Philadelphia (ANSP), Natural History Museum, London (BMNH), California Academy of Sciences, San Francisco (CAS), Museu de Ciências e Tecnologia, Pontifícia Universidade Católica do Rio Grande do Sul, Porto Alegre (MCP), and Universidade Federal da Bahia, Salvador (UFBA).
Morphological data. The measurements followed Boeseman, (1968)Boeseman M. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zoologische Verhandelingen. 1968; 99:1–89., modified by Weber, (1985)Weber C. Hypostomus dlouhyi, nouvelle espèce de poissonchat cuirassé du Paraguay (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool. 1985; 92:955–68. and Zawadzki et al., (2013)Zawadzki CH, Oliveira RRD, Debona T. A new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the rio Tocantins-Araguaia basin, Brazil. Neotrop Ichthyol. 2013; 11(1):73–80. https://doi.org/10.1590/S1679-62252013000100008
https://doi.org/10.1590/S1679-6225201300...
, 2020Zawadzki CH, Renildo RDO, Oliveira ADS, Py-Daniel LR. Redescription of Hypostomus carinatus (Steindachner 1881) (Siluriformes: Loricariidae) from the rio Amazonas basin in Brazil. Zootaxa. 2020; 4750(2):191–203. https://doi.org/10.11646/zootaxa.4750.2.3
https://doi.org/10.11646/zootaxa.4750.2....
). Nomenclature of plates and bone counts followed Schaefer, (1987)Schaefer SA. Osteology of Hypostomus plecostomus (Linnaeus), with a phylogenetic analysis of the loricariid subfamilies (Pisces: Siluroidei). Contrib Sci. 1987; 394:1–31., modified by Oyakawa et al., (2005)Oyakawa OT, Akama A, Zanata AM. Review of the genus Hypostomus Lacépède, 1803 from rio Ribeira de Iguape basin, with description of a new species (Pisces, Siluriformes, Loricariidae). Zootaxa. 2005; 921:1–27.. Standard length (SL) was expressed in millimeters and the other measurements in percentage of SL.
Morphological data analyses. Fifty-three preserved specimens were used in the morphological analysis, further confirmed to be distinct on the basis of molecular analysis: 15 of H. johnii, 10 of Hypostomus pusarum (Starks, 1913Starks EC. The fishes of the Stanford Expedition to Brazil. Stanford, Leland Stanford Junior University Publications. 1913. 77p.), 11 of Hypostomus aff. pusarum and 17 from Hypostomus sp. 2 (sensuRamos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
). Due to the low number of specimens of H. vaillanti (n = 4) the species was not included in the morphometric statistical analysis. We used the morphometric data of these specimens to perform discriminant analyses. Firstly, we corrected the allometric effect following Lleonart et al. (2000)Lleonart J, Salat J, Torres GJ. Removing allometric effects of body size in morphological analysis. J Theor Biol. 2000; 205(1):85–93. https://doi.org/10.1006/jtbi.2000.2043
https://doi.org/10.1006/jtbi.2000.2043...
to eliminate collinearity (Zuur et al., 2010Zuur A, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010; 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
https://doi.org/10.1111/j.2041-210X.2009...
). Then, we executed the VIF (variance inflation factor), removing some variables so that all 15 remaining ones presented a value above the threshold of five, in order to reduce the collinearity levels (Zuur et al., 2010Zuur A, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010; 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
https://doi.org/10.1111/j.2041-210X.2009...
).
Afterwards, aiming to decrease the data dimensionality (Jolliffe et al., (2002)Jolliffe IT, Uddin M, Vines SK. Simplied EOFs-Three alternatives to rotation. Clim Res. 2002; 20:271–79. https://www.int-res.com/articles/cr2002/20/c020p271.pdf
https://www.int-res.com/articles/cr2002/...
, the principal component analysis (PCA) was performed from the dataset of morphometric measurements with uncorrelated measurements, to characterize the variation of the samples and associate specimens with groups based on their characteristics (Schlager, 2017Schlager S. Morpho and Rvcg - shape analysis in R: R-packages for geometric morphometrics, shape analysis and surface manipulations. In: Zheng G, Li S, Székely G, editors. Statistical shape and deformation analysis: methods, implementation and applications. Academic Press; 2017. p.217–56.). Canonical variable analysis was also performed, with the aim of increasing the distances between groups (Mitteroecker, Bookstein, 2011Mitteroecker P, Bookstein F. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evolutionary Biology. 2011; 38(1):100–14. https://doi.org/10.1007/s11692-011-9109-8
https://doi.org/10.1007/s11692-011-9109-...
). These analyses were done using the package ggord (Beck, 2017Beck MW. ggord: Ordination Plots with ggplot2. R package version 0.11, 2017. https://zenodo.org/badge/latestdoi/35334615.
https://zenodo.org/badge/latestdoi/35334...
) in R software (R Development Core Team, 2018R Development Core Team. R: The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.r-project.org/
https://www.r-project.org/...
). Finally, we did a PERMANOVA as a discriminant analysis using the Vegan package, also in R software (Oksanen et al., 2019Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. vegan: Community Ecology Package (R package version 2.5-6). 2019.), in order to test whether there are significant differences (p <0.05) between the general means of the species, so that there is support for the morphological differences between the groups.
Molecular data. Of the four possible new species in relation to the morphotypes Hypostomus sp. 1, Hypostomus sp. 2, Hypostomus sp. 3 and Hypostomus sp. 4 proposed by Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, we were only not able to obtain tissues of Hypostomus sp. 3, which is a single individual. The sequences of H. pusarum were from specimens collected in Ceará-Mirim River, the species’ type-locality (Starks, 1913Starks EC. The fishes of the Stanford Expedition to Brazil. Stanford, Leland Stanford Junior University Publications. 1913. 77p.), because samples of H. pusarum from the Parnaíba river were not available, and this species was identified based on morphological characters.
DNA extraction was accomplished through the saline extraction protocol (modified from Bruford et al., 1992Bruford MW, Hanotte O, Brookfield JFY, Burke TA. Multilocus and single-locus DNA fingerprinting. In: Hoelzel CAR. Editor. Molecular Genetic Analysis of Populations. Oxford University Press. 1992; p.225–69.) as it follows: lysis buffer (Tris 1M Ph8, NaCl 5M, EDTA 0.5M Ph8, SDS 10%, Proteinase K 10mg/Ml, and dH2O), cold isopropanol precipitation, resuspension in Milli-Q water, and DNA storage in a -20°C freezer.
For the amplification of the gene cytochrome oxidase subunit I (cox1) through a polymerase chain reaction (PCR), we used the primers FishF1: 5’ TCAACCAACCACAAAGACATTGGCAC 3’ and FishR1: 5’ TAGACTTCTGGGTGGCCAAAGAATCA 3’ (Ward et al., 2005Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia’s fish species. Phil Trans R Soc B. 2005; 360(1462):1847–57. https://doi.org/10.1098/rstb.2005.1716
https://doi.org/10.1098/rstb.2005.1716...
). The program comprised a denaturation of 4 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 47°C, and 1 min at 72°C, finalizing with an extension phase of 5 min at 72°C. The PCR products were observed in 1% agarose gel dyed with GelRed (Biotium). Purification and sequencing steps of both forward and reverse strands were done by the Korean company Macrogen (http://www.macrogen.com).
We edited electropherograms using the software SeqMan (DNASTAR, Lasergene Software Package) and aligned the sequences in MEGA 6.0 (Tamura et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis, version 6.0. Mol Biol Evol. 2013; 30(12):2725–29. https://doi.org/10.1093/molbev/mst197
https://doi.org/10.1093/molbev/mst197...
) using the ClustalW (Thompson et al., 1994Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22:4673–80. https://doi.org/10.1093/nar/22.22.4673
https://doi.org/10.1093/nar/22.22.4673...
) algorithm. The final dataset has cox1 fragments of 651 base pairs.
Phylogenetic analyses and delimitation of lineages. We executed a Bayesian phylogenetic reconstruction in the software BEAST 1.8 (Drummond et al., 2012Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29:1969–73. https://doi.org/10.1093/molbev/mss075
https://doi.org/10.1093/molbev/mss075...
) using the nucleotide substitution model HKY + G, obtained in the software JModelTest 2.1.7 through the Bayesian Information Criterion (BIC). Divergence time among lineages was also calculated using BEAST 1.8 with a relaxed mutation rate of 0.29% per million years calibrated to Hypostomus (Silva et al., 2016Silva GSC, Roxo FF, Lujan NK, Tagliacollo VA, Zawadzki CH, Oliveira C. Transcontinental dispersal, ecological opportunity and origins of an adaptive radiation in the Neotropical catfish genus Hypostomus (Siluriformes: Loricariidae). Mol Ecol. 2016; 25(7):1511–29. https://doi.org/10.1111/mec.13583
https://doi.org/10.1111/mec.13583...
). The analysis was set to 106 MCMC (Markov Chain Monte Carlo) runs, and trees were sampled every 1,000 steps, adding up to 10,000 trees in each run. The visualization of Markov chains convergence was done in Tracer 1.6 (Drummond et al., 2012Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29:1969–73. https://doi.org/10.1093/molbev/mss075
https://doi.org/10.1093/molbev/mss075...
). We used a burn-in of 15,000 (15% of 10,000,000 trees saved every 1,000) and observed the nodes’ posterior probability values in Tree-Annotator v.1.8.1. (Drummond et al., 2012Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29:1969–73. https://doi.org/10.1093/molbev/mss075
https://doi.org/10.1093/molbev/mss075...
). Genetic distance calculations were done in MEGA 6.0 using the model K2P.
Five lineage delimitation analyses were carried out aiming to test if the morphological identifications agree with molecular ones. The Generalized Mixed Yule-Coalescent (GMYC, Fujisawa, Barraclough, 2013Fujisawa T, Barraclough TG. Delimiting species using single-locus data and the generalized mixed yule coalescent approach: A revised method and evaluation on simulated data sets. Syst Biol. 2013; 62(5):707–24. https://doi.org/10.1093/sysbio/syt033
https://doi.org/10.1093/sysbio/syt033...
) was done with single (sGMYC) and multiple (mGMYC) thresholds through the upload of an ultrametric tree from BEAST 1.8. Both the Bayesian implementation of Poisson tree processes (bPTP) and the Maximum Likelihood (mPTP) (Zhang et al., 2013Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013; 29(22):2869–76. https://doi.org/10.1093/bioinformatics/btt499
https://doi.org/10.1093/bioinformatics/b...
) were executed from the upload of a Maximum Likelihood tree with branch lengths proportional to nucleotides substitution from the software MEGA 6.0. For GMYC and PTP analyses, we used the online server www.species.h-its.org. Finally, the Automatic Barcode Gap Discovery (ABGD), which consists of the calculation of genetic distances from a unique locus, uses a distance matrix from MEGA 6.0 uploaded to the online server https://bioinfo.mnhn.fr/abi/public/abgd.
We used sequences of Hypostomus sertanejo Zawadzki, Ramos & Sabaj, 2017 from the Jaguaribe River, MNCE ecoregion, and of Hypostomus velhochico Zawadzki, Oyakawa & Britski, 2017 from the São Francisco River as comparative material, and a sample tissue of Parotocinclus spirulus (Fowler, 1941) as an outgroup (Tab. S1 ).
RESULTS
Three valid Hypostomus species were collected in Parnaíba River basin: H. johnii, H. pusarum and H. vaillanti making this the first record of H. pusarum in the MAPE ecoregion. Additionally, we analyzed four new possible species regarding the morphotypes Hypostomus sp. 1, Hypostomus sp. 2, Hypostomus sp. 3, and Hypostomus sp. 4 from Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
. However, our morphological analyses indicated that Hypostomus sp. 1 and Hypostomus sp. 4 from Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
are a single species, and its morphology is remarkably similar to H. pusarum. However, considering the remarkable genetic distance and some morphologic differences it is here mentioned as H. aff. pusarum. Hypostomus sp. 3 is a distinct morphotype as it has one additional dorsal-fin ray II+8 (vs. I+7) in the other species of the basin. Therefore, additional specimens, including for molecular analysis, are needed to verify if this species is an undescribed species. Meanwhile, we corroborated Hypostomus sp. 2 as new species both through morphological and molecular data, and it is described below. Thus, to solve the remaining taxonomic uncertainties, we need more samples and complementary analyses of both Hypostomus aff. pusarum and Hypostomus sp. 3.
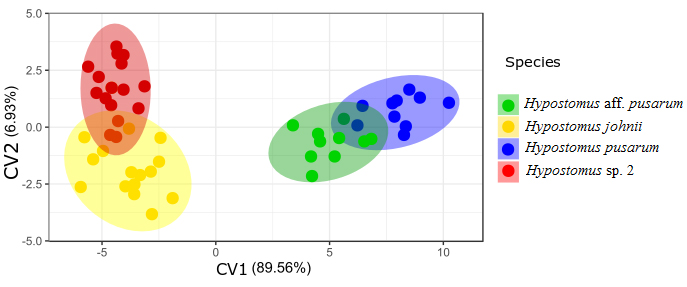
Morphological data. The measurements of 53 specimens of Hypostomus resulted in the CVA plot (Fig. 1), both axis CV1 and CV2 explained, together, 96.49% of the variation, with the measurements of cleithral width, head length, head height, interorbital distance, and maxillary length presented the highest values in CV1, and caudal peduncle height, orbital width, snout length, interdorsal length, and postanal length of caudal peduncle the highest ones in CV2. The PERMANOVA analysis was significant (p < 0.01) with respect to differences between the mean measurements of the analyzed lineages.
Canonical Variable Analysis (CVA) with morphological data of Hypostomus species from the Parnaíba River basin.
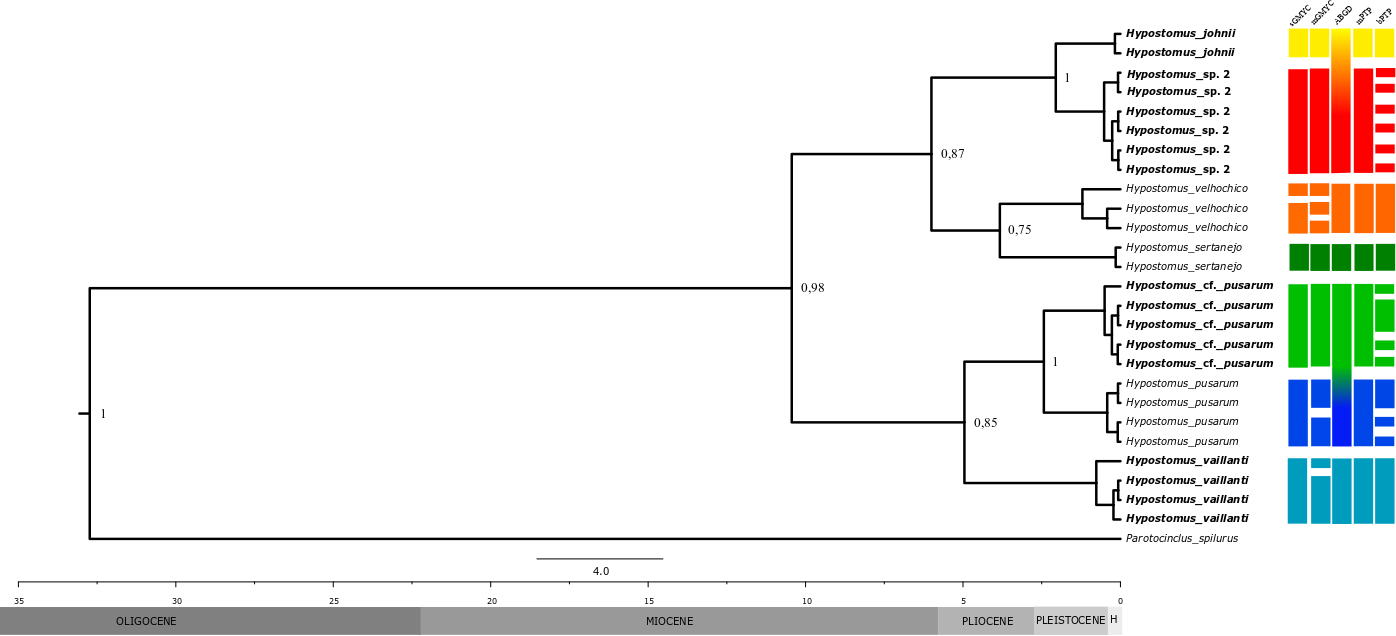
Molecular data. Tissue samples of 26 specimens of Hypostomus were used. In addition to species from the Parnaíba River, some species from the surrounding ecoregions were also sequenced (H. sertanejo and H. pusarum from the MNCE and H. velhochico from the SFRE). Based on the given topology by the Bayesian Inference, Hypostomus sp. 2 and H. johnii are a sister-clade to H. sertanejo from MNCE and H. velhochico from SFRE (posterior probability: 0.87), while H. pusarum and Hypostomus aff. pusarum are closely related to H. vaillanti. Given the molecular clock, we estimate that Hypostomus sp. 2 has diverged from its sympatric congener (H. johnii) about 2-3 Ma, at the end of the Pliocene and beginning of the Pleistocene (Fig. 2).
Phylogenetic tree with Bayesian inference for the mitochondrial cox1 gene, including species of Hypostomus that occur in the Parnaíba River (in bold), and other comparative species of the surrounding ecoregions: (H. pusarum and H. sertanejo from the Mid-Northeastern Caatinga) and H. velhochico from São Francisco ecoregion. The numbers above the branches are later probability values. H: Holocene.
Genetic distances (Kimura 2 parameters) between species of the genus Hypostomus from the Parnaíba River basin (Maranhão-Piauí ecoregion), and some species of the São Francisco and Mid- Northeastern Caatinga ecoregions. Bold diagonal numbers refer to intraspecific distances.
The genetic distance among all Hypostomus species varied from 1.2% (between H. johnii and Hypostomus sp. 2) to 5.5% (Hypostomus sp. 2 and H. pusarum) (Tab. 1). Most lineages’ delimitation analyses (sGMYC, mGMYC, and mPTP) considered Hypostomus sp. 2, H. johnii, Hypostomus aff. pusarum, H. pusarum, and H. vaillanti as distinct evolutionary units (Fig. 2).
Therefore, the agreements among distinct data sources, including the identification through traditional morphological characters, and the ancient and complete split of species from Parnaíba and adjacent ecoregions, confirm that Hypostomus sp. 2, from Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, is indeed a new species, described below.
In addition, the traditional morphology was used to elaborate an identification key for the Hypostomus species of the MAPE ecoregion. Based on this key, it will be possible to increase the knowledge of this group, which, due to its distinct morphological, ecological and molecular differences, including species in sympatry, with wide or restricted distribution, is a good model to test eco-evolutionary and biogeographic hypotheses.
Remarks.Hypostomus sp. 3 corresponds to one of the morphotypes proposed by Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, that until this study, only one specimen was collected in the Guaribas River, in Piauí State. Unlike other species of Hypostomus, Hypostomus sp. 3 has eight dorsal fin rays. This specimen may represent an anatomical malformation or a rare species; however, our sampling efforts were not enough to obtain more specimens of this morphotype.
Hypostomus velhomonge, new species
urn:lsid:zoobank.org:act:5E1C169B-CA24-460F-8513-BAF6324899CA
Hypostomus sp. 2. —Ramos et al., 2014Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
(listed, ichthyofauna of the Parnaíba River). —Silva et al., 2015Silva MJ, Costa BG, Ramos TPA, Auricchio P, Lima SMQ. Ichthyofauna of the Gurgueia river, Parnaíba river basin, northeastern Brazil. Check List. 2015; 11(5):1–8. https://doi.org/10.15560/11.5.1765
https://doi.org/10.15560/11.5.1765...
(listed, ichthyofauna of the Gurgueia River). —Lima et al., 2017Lima SMQ, Ramos TPA, Silva MJ, Rosa RS. Diversity, distribution, and conservation of the caatinga fishes: advances and challenges. In: Silva JMC, Leal IR, Tabarelli M., editors. Caatinga. Springer; 2017. p.97–131. (listed, ichthyofauna of the Caatinga).
Hypostomus velhomonge, UFPB 9565, holotype, 118.0 mm SL, rio Balsas, tributary of Parnaíba River, Maranhão State, Brazil.
Holotype. UFPB 9565, 118.0 mm SL, Brazil, Maranhão State, Sambaíba, rio Balsas, tributary of Parnaíba River, 07°08’33”S 45°20’45”W, 8 Feb 2009, T. P. A. Ramos, P. Charvet, R. T. C. Ramos, G. Moro & E. França.
Paratypes. All from Brazil. Parnaíba River basin, Maranhão State. MZUSP 5068, 3, 23.5–98.7 mm SL, Grajaú, rio Grajaú, 15 Jun 1966, Department of Zoology Tour. MZUSP 98591, 1, 81.2 mm SL, Balsas, rio Balsas, Parnaíba River basin, 07°32’13”S 46°02’20”W, O. T. Oyakawa, A. Akama, V. Garutti & J. C. Nolasco. NUP 15658, 2, 100.3–108.9 mm SL, collected with holotype. MNRJ 52967, 5, 51.9–89.2 mm SL, Alto Parnaíba, Parnaíba River, 09°06’53.8”S 45°55’37.8”W, 6 Feb 2009, T. P. A. Ramos, P. Charvet, R. T. C. Ramos, G. Moro & E. Franca. UFPB 7444, 10, 51.9–89.2 mm SL, Alto Parnaíba, Parnaíba River, 09°06’53.8”S 45°55’37.8”W, 6 Feb 2009, T. P. A. Ramos, P. Charvet, R. T. C. Ramos, G. Moro & E. Franca. UFPB 8250, 19, 24.6–91.9 mm SL, Alto Parnaíba, Parnaíba River, 09°06’52”S 45°55’35”W, 1 Apr 2010, T. P. A. Ramos & S. A. Q. A. Ramos. UFPB 9567, 4, 70.3–96.3 mm SL, Alto Parnaíba, Parnaíba River, 09°06’52”S 45°55’35”W, 24 Apr 2011, T. P. A. Ramos & S. A. Q. A. Ramos. Piauí State. NUP 15948, 11, 45.0–85.5 mm SL, Alto Parnaíba, at divide to Santa Filomena in Piauí State, Parnaíba River, 09°05’21.4”S 45°55’32.26”W, 9 Aug 2013, C. H. Zawadzki, T. Debona & D. Baumgartner. UFPB 9646, 21, 77.7-107.0 mm SL, collected with holotype. UFPB 8015, 1, 61.70–91.2 mm SL, Santa Filomena, Parnaíba River, 09°08’04.2”S 45°55’45.2”W, 15 Sep. 2009, T. P. A. Ramos, M. J. Silva & G. Moro.
Non-types. All from Brazil. Parnaíba River basin, Maranhão State. UFPB 8252, 5, 53.5–70.3 mm SL, São Francisco do Maranhão, Parnaíba River, 06°15’02”S 42°51’19”W, 6 Apr 2010, T. P. A. Ramos & S. A. Q. A. Ramos. UFPB 9547, 1, 71.3 mm SL, Alto Parnaíba, Barra do Brejo village, Parnaíba River, 09°18’16.3”S 45°54’13.0”W, 5 Feb 2009, T. P. A. Ramos, P. Charvet, R. T. C. Ramos, G. Moro & E. Franca. UFPB 9548, 2, 13.1–15.1 mm SL, Cachoeira stream, São Raimundo das Mangabeiras, tributary of Balsas River, 07°2’00.1”S 45°27’52”W, 8 Feb 2009, T. P. A. Ramos, P. Charvet, R. T. C. Ramos, G. Moro & E. Franca. UFRN 5514, 63.71–83.03 mm SL, Muquem stream, Manga, Barão do Grajaú, S. Costa, L. Neto, T. Ramos & Y. Ponce, 10 Dec 2018. UFRN 3092, 23, 14.51–84.50 mm SL, Parnaíba River, Alto Parnaíba, 09°06’54.3”S 45°55’37.8”W, 23 Jun 2014, S. M. Q. Lima, T. P. A. Ramos & M. J. Silva. Piauí State. MNRJ 42593, 3, 61.7–91.2 mm SL, Santa Filomena, Parnaíba River, 09°30’45.3”S 45°20’39.2”W, 15 Sep 2009, T. P. A. Ramos, M. J. Silva & G. Moro. MZUSP 5125, 1, 64.9 mm SL, Teresina, Parnaíba River, 19 Jun 1966, Department of Zoology Tour. MZUSP 74921, 4, 71.6–91.2 mm SL, rio Sorubim, tributary to rio Longá, 17 Nov 2001, M. C. C. De Pinna. NUP 15956, 1, 67.9 mm SL, Corrente, rio Corrente, 10°26’29.4”S 45°10’23.2”W 12 Aug 2013, C. H. Zawadzki & D. Baumbartner. UFPB 8249, 11, 39.9–81.7 mm SL, Santa Filomena, Parnaíba River, 09°08’04.2”S 45°55’45.2”W, 2 Apr 2010, T. P. A. Ramos and S. A. Q. A. Ramos. UFPB 9549, 2, 52.5–66.6 mm SL, Ribeiro Gonçalves, Parnaíba River, 07°33’24.6”S 45°14’58.2”W, Jul 2005, W. Severi. UFPB 9550, 2, 33.4–57.8 mm SL, Amarante, Parnaíba River, 06°14’36.3”S 42°51’24.7”W, Jul 2005, W. Severi. UFPB 9566, 44, 51.3-85.6 mm SL, Santa Filomena, Parnaíba River, 09°08’04.2”S 45°55’45.2”W, 21 Oct 2010, T. P. A. Ramos & S. A. Q. A. Ramos. UFPB 9568, 1, 82.1 mm SL, Ribeiro Gonçalves, Parnaíba River, 07°33’24.6”S 45°14’58.2”W, 23 Apr 2011, T. P. A. Ramos & S. A. Q. A. Ramos. UFRN 2720, 3, 53.9–86.8 mm SL, Rio Corrente, Corrente, 10°25’30.6”S 45°11’47.4”W, 18 Apr 2014, T. Ramos, L. Neto, M. Germano & L. Medeiros. UFRN 2806, 1, 66.5 mm SL, headwaters of Rio Gurguéia, São Gonçalo do Gurguéia, 10°4’38.8”S 45°20’18.2”W, 19 Jun 2014, S. Lima, R. Paiva, M. Silva & Y. Ponce. UFRN 3006, 1, 82.6 mm SL, Rio Uruçuí Vermelho, Barreiras do Piauí, 09°58’53”S, 45°33’11.7”W, 21 Jun 2014, S. Lima, T. Ramos and M. Silva.
Diagnosis.Hypostomus velhomonge differs from congeners, except Hypostomus alatus Castelnau, 1855, H. arecuta Cardoso, Almirón, Casciotta, Aichino, Lizarralde & Montoya-Burgos, 2012, H. bolivianus (Pearson 1924), H. denticulatus Zawadzki, Weber & Pavanelli, 2008, H. francisci (Lütken, 1874), H. freirei Penido, Pessali & Zawadzki, 2021, H. isbrueckeri Reis, Weber & Malabarba, 1990, H. jaguar Zanata, Sardeiro & Zawadzki, 2013, H. kuarup Zawadzki, Birindelli & Lima, 2012, H. leucophaeus Zanata & Pitanga, 2016, H. luteomaculatus (Devincenzi, 1942), H. meleagris (Marini, Nichols & LaMonte 1933), H. multidens Jerep, Shibatta & Zawadzki, 2007, H. mutucaeKnaack, 1999Knaack J. New Ancistrus species from the Rio Cuiba system, Brazil (Pisces, Siluriformes, Loricariidae). TFH Magazine. 1999; 47:150–55., H. myersi (Gosline, 1947), H. Paulinus (Ihering, 1905), H. regani (Ihering, 1905), H. strigaticeps (Regan, 1908), H. ternetzi (Boulenger, 1895), H. unae (Steindachner, 1878), H. uruguayensis Reis, Weber & Malabarba, 1990, and H. yaku Martins, Langeani & Zawadzki, 2014 by having high number of teeth on premaxillary (62–95, mode 73) and dentary (64–110, mode 76) (vs. smaller number of teeth on both premaxillary and dentary, rarely more than 50). Hypostomus velhomonge differs from H. alatus, H. arecuta, H. francisci, H. luteomaculatus, H. meleagris, H. multidens, H. myersi, H. regani, H. sertanejo and H. strigaticeps by having dark spots over body and fins (vs. pale spots). The new species is distinguished from H. bolivianus, H. denticulatus, H. freirei, H. isbrueckeri, H. jaguar, H. johnii, H. ternetzi, H. uruguayensis and H. leucophaeus by having ventral region of head and anteromedial region of abdomen naked, even on larger specimens (vs. ventral region of head and anteromedial region of abdomen covered by plates at least on larger specimens). Additionally, H. velhomonge differs from H. bolivianus by having bicuspid teeth (vs. unicuspid teeth); from H. denticulatus by having teeth with asymmetric cusps (vs. teeth with symmetrical cusps); from H. isbrueckeri by having homogeneous caudal-fin ground color, without marks (vs. yellow band on distal caudal-fin margin in mature males); from H. jaguar by possessing faint, small dark spots on head, the spots, smaller than the pupil (vs. head covered with large spots, larger than the pupil); from H. johnii by presenting the caudal-fin ventral lobe slightly longer than dorsal lobe (vs. ventral lobe of caudal-fin much longer than dorsal lobe); from H. ternetzi by having roughly flat interorbital and predorsal region (vs. interorbital and predorsal region with strong median keel); from H. unae by having compressed caudal peduncle, its depth larger than its width at adipose-fin origin (vs. rounded caudal peduncle, its depth equal to its width at adipose-fin origin); from H. vaillanti by having ventral region of head and abdomen usually without spots; dark spots present in few specimens (vs. ventral region of head and abdomen with conspicuous spots in the shape of whole or half rings,); from H. uruguayensis by having one predorsal plate bordering supraoccipital (vs. three predorsal plates bordering supraoccipital). The new species can be distinguished from Hypostomus kuarup by having large dark spots on sides of body, spots similar in length to eye diameter (vs. small dark spots, similar in length to pupil diameter); from H. mutucae by having narrower dentaries, each dentary length about half interorbital width (vs. similar in length to interobital width; see figs. in p. 103 and 106 of Knaack, 1999Knaack J. New Ancistrus species from the Rio Cuiba system, Brazil (Pisces, Siluriformes, Loricariidae). TFH Magazine. 1999; 47:150–55. and fig. 7 in Zawadzki et al., 2012Zawadzki CH, Birindelli JLO, Lima FCT. A new armored catfish species of the genus Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the upper rio Xingu basin, Brazil. Neotrop Ichthyol. 2012; 10(2):245–53. https://doi.org/10.1590/S1679-62252012000200003
https://doi.org/10.1590/S1679-6225201200...
), by having smaller angle between dentaries, from 115° to 135° (vs. from 160° to 170°), and by having round oral disk (vs. clearly transversally elongated); from H. yaku by lacking hypertrophied odontodes on flanks (vs. hipertrophied odontodes on flanks, more developed in mature males).
Description. Meristic and morphometric data are shown in Tab. 2. Largest specimen examined 120.9 mm SL. Body moderately depressed. Greatest body width at cleithral region, progressively tapering to end of caudal peduncle. Dorsal profile raising slightly convex from tip of snout to dorsal-fin origin; some individuals with a straight area between supraoccipital posterior portion and dorsal-fin origin; slightly concave from dorsal-fin origin to region of dorsal procurrent caudal-fin rays. Head and snout wide and moderately depressed. Head depth approximately half its width at eyes; dorsal region of head totally covered by dermal bones with odontodes. Ventral region of head, scapular bridge and abdomen mostly naked, except for some dispersed few plates at anterior branchial opening, scapular bridge and some plates around pectoral-fin origin and on preanal region in specimens about 100 mm SL (Fig. 4). Inconspicuous bulge formed by mesethmoid from snout tip to upper region between nostrils. Orbit narrow, dorsolaterally positioned, 14.7–18.7% in head length. Supraoccipital with median crest. Predorsal region with three unpaired plates and slightly divergent keel. Dorsal fin II,7; origin slightly anterior to vertical pelvic-fin insertion; dorsal fin when depressed almost to just reaching adipose-fin origin; distal border slightly convex. Pectoral fin I,6; spine long and depressed, reaching 1/3 to half pelvic-fin unbranched ray length, and covered by hypertrophied odontodes on distal region (more developed in larger specimens); distal margin straight. Pelvic fin i,5; unbranched ray long, depressed and slightly curved inward reaching anal-fin insertion. Anal fin, i,5; extending to sixth plate after its origin; distal border slightly thin. Caudal fin i,14,i; emarginate, with ventral unbranched ray equal to slightly longer than dorsal unbranched ray.
Body covered by five lateral series of plates. Dorsal series with keel from its origin to adipose fin. Mid-dorsal series without keel. Median series lacking keel. Median series bearing complete lateral line, with 25 to 26 plates. Mid-ventral series strongly bent until fourth or fifth plates. Ventral series slightly bent to form straight caudal peduncle roof. Tip of snout covered by odontodes, except for a central area similar in size to eye diameter. Adipose fin straight and oriented approximately 30° backward from dorsal surface of caudal peduncle.
Dorsal and lateral region of trunk covered by three predorsal plates and by five longitudinal series of dermal plates. Dorsal series origin below dorsal-fin spine, bent until adipose-fin insertion. Mid-ventral series without keels. Median series with origin at posterior region of scapular bridge, devoid of keels and supporting continuous lateral line. Mid-ventral series with first four to five plates bent. Ventral series bent ventrally. Oral disk round, with numerous small papillae; papillae larger proximally to mouth. Mouth without hypertrophied medial buccal papilla. Maxillary barbel slightly smaller than eye pupil. Dentaries moderately lengthened, angling approximately 115° to 135° to each other. Premaxilla with 62 to 95, and dentary with 64 to 110 teeth; all teeth slender and bifid with internal cusps shorter than external ones. Crowns bent inward mouth.
Ontogenetic development of plates from the anteromedial region of Hypostomus johnii and H. velhomonge. A. Hypostomus velhomonge, UFPB 8015, 73.58 mm SL; B. H. johnii UFPB 9535, 71.81 mm SL; C. H. velhomonge, UFPB 8015, 90.26 mm SL; D. H. johnii UFPB 9535, 90.54 mm SL; E. H. velhomonge, UFPB 9565, 118.29 mm SL; F. H. johnii UFPB 9267, 114.28 mm SL.
Coloration in alcohol. Predominant general color light brown. Usually faded dark spots on anterior region of body, larger backwards. Over the head, faded dark, round, close together spots present, with maximum size similar to the pupil, most of them smaller; spots less visible anterior to eyes. Towards posterior portion of body spots reaching similar size to eye diameter, mainly at caudal peduncle region; the spots form somewhat diagonal rows from dorsal to ventral surface, including caudal peduncle. On trunk, some specimens without visible spots. Fins with dark, round spots on spines, soft rays and interradial membranes, predominantly over the latter. Caudal fin with elongate spots, predominantly located on spines and soft rays. Caudal fin spots transversely aligned to form series of concentric semicircles. Dorsal, pectoral and pelvic fins with spots aligned in somewhat irregular series parallel to rays. Spots over spines ranging from 2–3 series (pectoral fin) and from 1–2 (other fins). Abdominal region between pectoral and pelvic girdle, and around anus whitish; area corresponding to girdles light to very light brown; ventral surface of caudal peduncle light brown without spots.
Coloration in life. Color pattern of living specimens similar to individuals preserved in alcohol, except for more brownish gray color on body and fins and conspicuous dark spots in living specimens (Fig. 5).
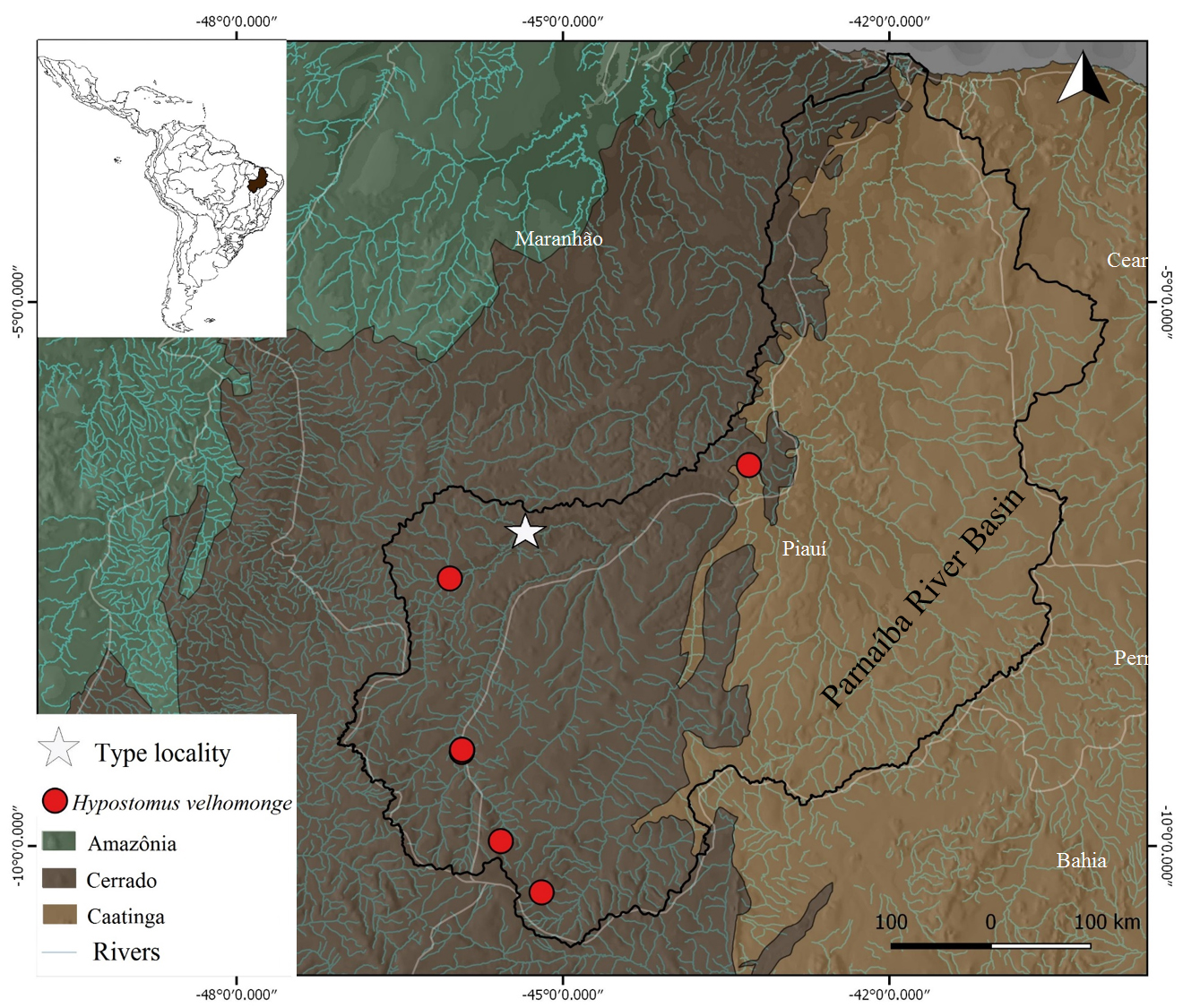
Geographical distribution.Hypostomusvelhomonge is apparently endemic to Parnaíba River basin and, so far, the species has only been found in the upper and middle portions of the basin, restricted to Cerrado areas in the drainage (Fig. 6).
Hypostomus velhomonge, color in life, UFPB 9566, paratype, 88.0 mm SL, with non-deposited material. Parnaíba River, Santa Filomena, Piauí, Brazil.
Geographic distribution of Hypostomus velhomonge in the Parnaíba River basin, Northeast Brazil.
Ecological notes and habitat.Hypostomusvelhomonge was recorded in co-occurrence with other loricariids such as H. vaillanti in the Muquém stream, in Barão de Grajaú, Maranhão, with Loricaria parnahybae Steindachner, 1907 and Loricariichthys derbyi Fowler, 1915 in the rio Balsas. All localities where Hypostomus velhomonge were recorded presented riparian forest typical of the Cerrado biome (Fig. 6). The type locality, Balsas River, has clear waters, rocky and sandy substrate and varying amounts of remnants of riparian vegetation.
Etymology. The specific epithet, “velhomonge”, is a reference to the Parnaíba River, commonly known as ‘Velho Monge’ (Old Monk, in English). One of the versions on the origin of this name portrays that a poet called Costa e Silva gave the river the nickname “Velho Monge” because, when seen from the city of Amarante, the confluence of Canindé River with Parnaíba River forms a landscape that, in profile, reminds the silhouette of a monk and whose foam suggests its long beard. A noun in apposition.
Common names. Cari, acari, bodó.
Conservation status.Hypostomusvelhomonge possesses a relatively broad distribution, occurring in the upper and middle portions of the Parnaíba River basin. Therefore, apparently does not match any of the extinction risk it is classified as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions committee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Prepared by the Standards and Petitions Committee; 2019. https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
Key to the species of Hypostomus from Maranhão-Piauí ecoregion
1a. Dark vermiculated color marks Hypostomus vaillanti (Fig. 7)
1b. Rounded dark spots 2
2a. Premaxilla and dentary, each with more than 100 teeth 3
2b. Premaxilla and dentary each with less than 100 teeth 4
3a. Anteromedial region covered by plates and odontodes and ventral
lobe of caudal-fin much longer than dorsal lobe Hypostomus johnii (Fig. 8)
3b. Anteromedial region deprived of plates and odontodes and the caudal-fin
ventral lobe slightly longer than dorsal lobe Hypostomus velhomonge (Fig. 3)
4a. Body and head covered with spots usually smaller
than the diameter of the eye Hypostomus pusarum (Fig. 9)
4b. Body and head covered with spots usually larger or the same size as the
diameter of the eye, conspicuous and spaced Hypostomus aff. pusarum (Fig. 10)
Hypostomus vaillanti, UFRN 5648, 240.2 mm SL. Parnaíba River, Barão de Grajaú, Maranhão, Brazil.
Hypostomus johnii, UFPB 9268, 107.7 mm SL. Poti River, tributary of the Parnaíba River basin, Crateús, Ceará, Brazil.
Hypostomus pusarum, UFPB 9265, 150.0 mm SL. Poti River, tributary of the Parnaíba River basin, Crateús, Ceará, Brazil.
Hypostomus aff. pusarum, UFPB 11077, 164.5 mm SL, Ingazeiro reservoir, Paulistana, Piauí, Brazil.
DISCUSSION
The integrative taxonomy of the Hypostomus species from the Parnaíba River basin allowed refining the taxonomy of this diverse Neotropical genus. Most of the species previously identified to genus level by Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
were identified, with the exception of Hypostomus aff. pusarum. The latter belongs to a confusing nomenclatural group of species and deserves an exclusive study to determine their validity and distribution. Although molecular samples of H. pusarum from the Parnaíba River basin were not available, our study indicated that Hypostomus sp. 1 and Hypostomus sp. 4 are indeed a single species (Hypostomus aff. pusarum). Our data also corroborated the status of a new species based both on morphology and molecular data, yielding the description of H. velhomonge. The new species occurs in the Parnaíba River basin with five herein confirmed congeners: H. johnii, H. pusarum, Hypostomus aff. pusarum, Hypostomus sp. 3, and H. vaillanti. The present study used only specimens of H. johnii measuring above 70 mm SL and the results differed from that described by Ramos et al., (2017)Ramos TPA, Zawadzki CH, Ramos RTDC, Britski HA. Redescription of Hypostomus johnii, a senior synonym of Hypostomus eptingi (Siluriformes: Loricariidae), Northeastern Brazil. Neotrop Ichthyol. 2017; 15(2):e160064. https://doi.org/10.1590/1982-0224-20160064
https://doi.org/10.1590/1982-0224-201600...
. Ramos et al., (2017)Ramos TPA, Zawadzki CH, Ramos RTDC, Britski HA. Redescription of Hypostomus johnii, a senior synonym of Hypostomus eptingi (Siluriformes: Loricariidae), Northeastern Brazil. Neotrop Ichthyol. 2017; 15(2):e160064. https://doi.org/10.1590/1982-0224-20160064
https://doi.org/10.1590/1982-0224-201600...
were not able to distinguish the two species. Here, in the light of the new data H. johnii was up to now be restricted to the lower and middle Parnaíba River portions in Ceará, Piauí, and Maranhão states, and endemic to this basin, occurring mainly in the Caatinga (Fig. 11). Hypostomus pusarum and H. vaillanti occur only in the middle portion of Parnaíba and Hypostomus aff. pusarum occur in all portions of the basin (Fig. 11). While H. velhomonge occurs in the upper stretches of the Parnaíba River basin, in the Cerrado biome.
Hypostomus species distribution map from the Parnaíba River basin, Northeast Brazil (except H. velhomonge, depicted in Fig.6), with biome delimitation.
Hypostomus velhomonge differs from all other species of the Parnaíba River basin by its naked anteromedial region of abdomen (vs. abdomen usually mostly covered by plates; some specimens with small naked areas between lateral plates and minor central plates, and at pectoral and pelvic insertions). The new species morphologically resembles its closely related species, H. johnii. This resemblance is due to the great number of dentary and premaxillary teeth, which are above 60, but it can be also easily distinguishable inconspicuous dark spots on H. johnii), and by having a smaller dentary angle, that varies from 115° to 135° (vs. 160° to 170° in H. johnii).
This similarity accounted for identification of H. velhomonge as H. johnii by Ramos et al., (2017)Ramos TPA, Zawadzki CH, Ramos RTDC, Britski HA. Redescription of Hypostomus johnii, a senior synonym of Hypostomus eptingi (Siluriformes: Loricariidae), Northeastern Brazil. Neotrop Ichthyol. 2017; 15(2):e160064. https://doi.org/10.1590/1982-0224-20160064
https://doi.org/10.1590/1982-0224-201600...
. These authors included in their analyses all individuals with more than 60 teeth counts per jaw ramus and with a wide dentary angle from the Parnaíba River basin. However, in specimens smaller than 70 mm SL, it is usual that plates on the anteromedial region of abdomen in H. johnii (main diagnostic character between H. velhomonge and H. johnii) are not yet developed (Fig. 4), and these two species were confounded as a single one. However, a wider head and cleithral region of H. johnii in relation to H. velhomonge was supported by the significance analysis with morphometric data, in which cleithral width and maxillary length were two of the measurements that contributed the most for the partial segregation of these species. Besides, the caudal peduncle height is smaller in H. velhomonge specimens, demonstrating that the new species is more dorsoventrally flattened than H. johnii.
The cox1 interspecific genetic distance between H. velhomonge and H. johnii is 1.2%, a value considered the mean for Hypostomus sister-species, according to De Queiroz et al., (2020)De Queiroz LJ, Cardoso Y, Jacot-des-Combes C, Bahechar IA, Lucena CA, Py-Daniel LR, Torrente-Vilara G. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus.Mol Phylogenet Evol. 2020; 145:106711. https://doi.org/10.1016/j.ympev.2019.106711
https://doi.org/10.1016/j.ympev.2019.106...
. Even though there is a genetic distance value for delimiting species with DNA barcode (~2%), they should be interpreted considering many aspects related to the speciation process among lineages, such as divergence time, reciprocal monophyly, and population size (Carvalho, 2011Carvalho PH. Análises filogenéticas e filogeográficas do complexo de espécies Hypostomus ancistroides (Siluriformes: Loricariidae). [Doctoral thesis]. São Paulo: Universidade de São Paulo; 2011.; Pereira et al., 2011Pereira LH, Maia GM, Hanner R, Foresti F, Oliveira C. DNA barcodes discriminate freshwater fishes from the Paraíba do Sul River Basin, São Paulo, Brazil. Mitochondrial Dna. 2011; 22:71–79. https://doi.org/10.3109/19401736.2010.532213
https://doi.org/10.3109/19401736.2010.53...
, 2013Pereira LH, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013; 14(20). https://doi.org/10.1186/1471-2156-14-20
https://doi.org/10.1186/1471-2156-14-20...
; Berbel-Filho et al., 2018Berbel-Filho WM, Ramos TPA, Jacobina UP, Maia DJG, Torres RA, Lima SMQ. Updated checklist and DNA barcode-based species delimitations reveal taxonomic uncertainties among freshwater fishes from the mid-north-eastern Caatinga ecoregion, North-Eastern Brazil. J Fish Biol. 2018; 93(2):311–23. https://doi.org/10.1111/jfb.13758
https://doi.org/10.1111/jfb.13758...
). Other authors reported that for Hypostomus the genetic distance among congeners tends to be below 2% (Pereira et al., 2013Pereira LH, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013; 14(20). https://doi.org/10.1186/1471-2156-14-20
https://doi.org/10.1186/1471-2156-14-20...
; Anjos et al., 2020Anjos MS, Bitencourt JA, Nunes LA, Sarmento-Soares LM, Carvalho DC, Armbruster JW, Affonso PR. Species delimitation based on integrative approach suggests reallocation of genus in Hypostomini catfish (Siluriformes, Loricariidae). Hydrobiologia. 2020; 847(2):563–78. https://link.springer.com/article/10.1007/s10750-019-04121-z
https://link.springer.com/article/10.100...
; De Queiroz et al., 2020De Queiroz LJ, Cardoso Y, Jacot-des-Combes C, Bahechar IA, Lucena CA, Py-Daniel LR, Torrente-Vilara G. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus.Mol Phylogenet Evol. 2020; 145:106711. https://doi.org/10.1016/j.ympev.2019.106711
https://doi.org/10.1016/j.ympev.2019.106...
). Other loricariid groups from Northeastern Brazil also show low interspecific genetic distances, such as Parotocinclus seridoensis Ramos, Barros-Neto, Britski & Lima, 2013 and P. spilurus (Fowler 1941) (Berbel-Filho et al., 2018Berbel-Filho WM, Ramos TPA, Jacobina UP, Maia DJG, Torres RA, Lima SMQ. Updated checklist and DNA barcode-based species delimitations reveal taxonomic uncertainties among freshwater fishes from the mid-north-eastern Caatinga ecoregion, North-Eastern Brazil. J Fish Biol. 2018; 93(2):311–23. https://doi.org/10.1111/jfb.13758
https://doi.org/10.1111/jfb.13758...
).
Nevertheless, the divergence time between H. johnii and H. velhomonge is approximately 2 Mya. Even though it has been discussed that Hypostomus species might have diversified in Late Miocene and Pleistocene (12 to 4 Ma) (Montoya-Burgos, 2003Montoya-Burgos JI. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol. 2003; 12(7):1855–67. https://doi.org/10.1046/j.1365-294X.2003.01857.x
https://doi.org/10.1046/j.1365-294X.2003...
; Pereira et al., 2013Pereira LH, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013; 14(20). https://doi.org/10.1186/1471-2156-14-20
https://doi.org/10.1186/1471-2156-14-20...
), our data suggest the most recent Pleistocene events could also have acted as a diversification driver for species that inhabit the northeastern Brazil drainages. Indeed, for seasonal rivulid fishes, speciation in this region occurred intensively during the Lower Pleistocene (Costa et al., 2018Costa WJEM, Amorim PF, Mattos JLO. Synchronic historical patterns of species diversification in seasonal aplocheiloid killifishes of the semi-arid Brazilian Caatinga. PloS ONE. 2018; 13(2). https://doi.org/10.1371/journal.pone.0193021
https://doi.org/10.1371/journal.pone.019...
).
Most species of Hypostomus from northeastern Brazil show dark spots on a lighter-colored body. The exceptions are H. vaillanti (MAPE) with dark vermiculations on a lighter body, H. alatus, H. francisci (São Francisco ecoregion), and H. sertanejo (MNCE) with yellowish spots on a dark brown colored body. The sister-species, H. johnii and H. velhomonge, show dark spots on a light color background, differing from H. sertanejo (MNCE), which has light spots on a dark body (Zawadzki et al., 2017Zawadzki CH, Ramos TPA, Sabaj M. Hypostomus sertanejo (Siluriformes: Loricariidae), new armoured catfish species from north-eastern Brazil. J Fish Biol. 2017; 91(1):317–30. https://doi.org/10.1111/jfb.13349
https://doi.org/10.1111/jfb.13349...
). However, all three species present morphological features in common, such as the elevated number of teeth, the widest dentary angles within Hypostomus of MAPE and MNCE ecoregions, besides more dorsoventrally flattened snouts.
Hypostomus velhomonge is distributed throughout the main course of the Parnaíba River basin, occurring on its the upper and middle portions. Some localities consisted in headwaters with higher velocity and dissolved oxygen, in an area with a high influence of the Cerrado (Silva et al., 2015Silva MJ, Costa BG, Ramos TPA, Auricchio P, Lima SMQ. Ichthyofauna of the Gurgueia river, Parnaíba river basin, northeastern Brazil. Check List. 2015; 11(5):1–8. https://doi.org/10.15560/11.5.1765
https://doi.org/10.15560/11.5.1765...
). Besides its apparent restriction to the Cerrado rivers, which is different from H. johnii that only registered in the Caatinga, the main feature of H. velhomonge is a naked anteromedial region since the initial developing stages to the maturity. This characteristic in Hypostomus was also attributed as a possible adaptation to fast-flowing environments (Zawadzki et al., 2012Zawadzki CH, Birindelli JLO, Lima FCT. A new armored catfish species of the genus Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the upper rio Xingu basin, Brazil. Neotrop Ichthyol. 2012; 10(2):245–53. https://doi.org/10.1590/S1679-62252012000200003
https://doi.org/10.1590/S1679-6225201200...
).
Hypostomus sp. 1 and Hypostomus sp. 4 of Ramos et al. (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
were identified by molecular and morphological analyses as the same taxonomic unit (Hypostomus aff. pusarum) and is morphologically similar to H. pusarum. However, genetically, this morphotype pertains to a distinct lineage of H. pusarum, with 1.5% of genetic distance from specimens of the type locality in MNCE, resembling that observed between H. johnii and H. velhomonge. Hypostomus aff. pusarum has some characteristics that differ from H. pusarum, such as the darker color, larger size and more spacing of the spots on the body and head of the specimens, in addition to the standard-length inferior to those of H. pusarum. Still, H. pusarum is a complex of nominal species morphologically similar to each other, with some of them synonyms (Berbel-Filho et al., 2018Berbel-Filho WM, Ramos TPA, Jacobina UP, Maia DJG, Torres RA, Lima SMQ. Updated checklist and DNA barcode-based species delimitations reveal taxonomic uncertainties among freshwater fishes from the mid-north-eastern Caatinga ecoregion, North-Eastern Brazil. J Fish Biol. 2018; 93(2):311–23. https://doi.org/10.1111/jfb.13758
https://doi.org/10.1111/jfb.13758...
), demanding a taxonomic revision before any species description. An integrative study concerning the type-series and wide samplings is in conclusion by the authors and should soon elucidate the confusing taxonomy of this complex. Seemingly, H. pusarum has a wide distribution in MNCE, also occurring in SAFR ecoregion (Zawadzki et al., 2017Zawadzki CH, Ramos TPA, Sabaj M. Hypostomus sertanejo (Siluriformes: Loricariidae), new armoured catfish species from north-eastern Brazil. J Fish Biol. 2017; 91(1):317–30. https://doi.org/10.1111/jfb.13349
https://doi.org/10.1111/jfb.13349...
). In Parnaíba River basin this species was collected up to now in three localities of the Poti River, in Caatinga area, close to the headwaters of MNCE’s rivers, and its occurrence could be the result of headwaters’ capture or even anthropic introduction. Nevertheless, no samples of this species from the Parnaíba basin were available for molecular analyses and only morphological characters were used for its identification (Fig. 10).
For a long time, the Parnaíba River basin was treated as one with low freshwater fish species richness and endemism. From the results obtained by Ramos et al., (2014)Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
https://doi.org/10.1590/S1676-0602014003...
, this view started to be questioned. Higher richness and endemism in Parnaíba River basin were confirmed for the Hypostomus catfishes, in a total of six species occurring in the Parnaíba River, three of them potentially endemic, including the possibility of other two new species. These results raised the knowledge of the group that, by presenting morphological, ecological, and molecular differences among sympatric species, consists in a great model for testing eco-evolutionary and biogeographic hypotheses.
Comparative material examined.Hypostomusalatus: UFRN 5509, 1, 88.7 mm SL Poção stream, Juazeiro, Bahia, Brazil; UFPB 3020, 1, 122.2 mm SL; UFPB 7459, 1, 121.1 mm SL, Bahia, Santana, Corrente River; NUP 9119, 1, 110.1 mm SL, Minas Gerais, Curimataí River; NUP 9829, 5, 139.0–77.4 mm SL, Minas Gerais, das Velhas River; NUP 9837, 4, 124.4–217.6 mm SL, Minas Gerais, Cipó River. Hypostomus brevicauda (Günther, 1864): BMNH 1864.1.19.16-17, 2, 189.0–196.1 mm SL, syntypes; MCP 36709, 3, 52.7–125.4 mm SL, Bahia, Camacã, Traíra stream; UFBA 0066, 1, 133.92 mm SL, Bahia, Xique-Xique, Suacica River, São Francisco basin; UFPB 4541, 15, 140.5–189.2 mm SL, Bahia, Una, Una River, Brazil 8 km SE of São José; UFPB 4563, 1, 166.9 mm SL, Bahia, Una, Aliança River. Hypostomus eptingi (Fowler, 1941): ANSP 69447, 122.1 mm SL, holotype, Ceará, Fortaleza; ANSP 69448, 2, 110.0–112.7 mm SL, paratype, same data as holotype; NUP 14886, 10, 37.2–80.9 mm SL, Ceará, Fortaleza, Cocó River; UFPB7698, 4, 80.1–87.2 mm SL, Ceará, Caucaia, Ceará River; UFPB 9220, 1, 101.51 mm SL, Ceará, Guaiuba, Baú River. Hypostomus franscici (Lütken, 1874): UFPB 3021, 4, 99.7–115.6 mm SL, Bahia, Santana, Corrente River; MCP 14038, 1, 180.0 mm SL, Minas Gerais, Três Marias reservoir; NUP 9940, 6, 111.0–187.1 mm SL; NUP 9945, 2, 148.6–150.7 mm SL, Minas Gerais, das Velhas River. Hypostomus garmanni (Regan, 1904): BMNH 1904.1.28.3, holotype, 209.9 mm SL, NUP 9819, 9, 87.7–204.2 mm SL; NUP 10028, 1, 78.8 mm SL; NUP 10031, 6, 136.6–170.2 mm SL, Minas Gerais, das Velhas River; UFPB 3018, 1, 89.0 mm SL, Bahia, Santana, Corrente River. Hypostomus gomesi (Fowler, 1942): ANSP 69409, 1, 141.4 mm SL, holotype, Ceará, Jaguaribe River basin. Hypostomus jaguar Zanata, Sardeiro & Zawadzki, 2013: NUP 4448, 2, 126.8–152.9 mm SL, Bahia, Itaberabá, Paraguaçu River; UFBA 6501, 8, 119.21–175.70 mm SL, Itaberabá, Paraguaçu River. Hypostomus jaguribensis (Fowler, 1915): UFPB 0053, 4, 40.6–68.1 mm SL; UFPB 0333, 22, 26.5–46.7 mm SL; UFPB 0341, 20, 40.6–68.1 mm SL; UFPB 0793, 1, 51.3 mm SL, Ceará, Barbalha, Salamandra River; UFPB 7699, 1, 101.2 mm SL, Ceará, Jaguaribe, Jaguaribe River basin; UFPB 7700, 6, 81.1–100.8 mm SL, Ceará, Icó, Salgado River; UFPB 9222, 1, 113.4 mm SL, Ceará, Itatira, Jaguaribe River basin; UFPB 9225, 15, 49.7–86.2 mm SL, Ceará, Lavras de Mangabeira, Machado River; UFPB 9226, 1, 140.5 mm SL, Ceará, Piquet Carneiro, Machado River; UFRN 0359, 17, 83, 20.2–85.7 mm SL, Ceará, Araripe, Paraíso reservoir; UFRN 0608, 8, 104.9–184.1 mm SL, Ceará, São Nicolau; UFRN 1168, 8, 72.7–83.7 mm SL, Lavras de Mangabeira, Cruzado stream; UFRN 1790, 2, 76.9–102.8 mm SL, Orós, Jaguaribe River; UFRN 1813, 1, 75.1 mm SL, Icó, Salgado River. Hypostomus johnii: UFRN 1341, 10, 10.49–64.61mm SL, Sambito River, Mesa de Pedra reservoir, Aroazes, Piauí, Brazil. UFRN 1233, 32, 13.90–93.51 mm SL, Sambito River, Mesa de Pedra reservoir, Aroazes, Piauí, Brazil. UFRN 2866, 3, 15.14–59.51 mm SL, Gurguéia River, São Gonçalo do Gurguéia, Piauí, Brazil. UFRN 2708,2, 35.84–59.78 mm SL, Tají River, Corrente, Piauí, Brazil. UFRN 3024, 5, 38.65–100.61 mm SL, Uruçuí-vermelho River, Barreiras do Piauí, Piauí. UFRN 2891, 8, 62.44–83.12 mm SL, Gurguéia River, São Gonçalo do Gurguéia, Piauí, Brazil. UFRN 1800, 6, 83.48–116.86 mm SL, Guaribas River, Picos, Piauí, Brazil. UFPB 3537, 9, 34.94–86.15 mm SL, Jacaraí River, Piracuruca, Piauí, Brazil. UFPB 9535, 18, 57.37–94.50 mm SL, Longá River, Nossa Senhora de Nazaré, Piauí, Brazil. UFPB 11078, 6, 43.71–67,51 mm SL, Gurguéia River, Jurumenha, Piauí, Brazil. UFPB 9267, 5, 92,48–129,12 mm SL, Poti River, Crateus, Ceará, Brazil. UFPB 9536, 12, 22.80–86.11 mm SL, Rio Longá, Parque ecológico Cachoeira do Urubu, Batalha, Piauí, Brazil. UFPB 9533, 1, 121,93 mm SL, São Nicolau River, Santa Cruz dos Milagres, Piauí, Brazil. Hypostomus lima (Lütken, 1874): BMNH 1876.1.10, 2, 72.9–86.1 mm SL, sintypes, Minas Gerais, Lagoa Santa; NUP5717, 4, 56.1–126.0 mm SL, Minas Gerais, ribeirão dos Patos; NUP 5721, 2, 47.5–72.8 mm SL, Minas Gerais, ribeirão das Minhocas; NUP 9827, 18, 81.5–181.5 mm SL, Minas Gerais, São Miguel. Hypostomus macrops (Eigenmann & Eigenmann, 1888): NUP 9831, 2, 97.7–106.8 mm SL; NUP 9832, 1, 172.6 mm SL, Minas Gerais, das Velhas River; NUP 9238, 1, 157.9 mm SL, Minas Gerais, Curimataí River. Hypostomus cf. margaritifer (Regan, 1908): UFPB 3019, 1, 97.1 mm SL; UFPB 3041, 1, 97.1 mm SL, Bahia, Corrente River, São Francisco basin. Hypostomus multidens: NUP 5340, 1, 157.0 mm SL, São Paulo, Piraju, Paranapanema River, Chavantes reservoir. Hypostomus mutucae: MCP 28669, 67.7 mm SL, holotype, Mato Grosso, Mutuca River, tributary of Paraguai River; NUP 6641, 13, 52.4–109.2 mm SL; NUP 6642, 4, 62.1–98.1 mm SL, Mato Grosso, Claro River, tributary of Paraguai River. Hypostomus nudiventris (Fowler, 1941): ANSP 69402, 56.8 mm SL, holotype; NUP 14687, 2, 78.5–100.3 mm SL, Ceará, Fortaleza, Choró River; UFPB 7697, 3, 78.3–118.4 mm SL, Ceará, Chorozinho, Choró River; UFPB 9223, 9, 61.8–74.5 mm SL, Itapiúna, Choró River. Hypostomus papariae (Fowler, 1941): ANSP 69398, 1, 94.3 mm SL, holotype; ANSP 69399, 1, 99.1 mm SL, paratype collected with the holotype; Rio Grande do Norte, Papary lake; ANSP 69400, 2, 102.7–126.6 mm SL, paratypes, Ceará, Fortaleza, Choró River; NUP 14684, 10, 54.6–104.4 mm SL, Rio Grande do Norte, Nísia Floresta, Ariri River; UFPB 7693, 32, 43.5–72.0 mm SL, Rio Grande do Norte, Nísia Floresta, Trairí River. Hypostomus paulinus: NUP 5344, 1, 69.0 mm SL, São Paulo, Passa Cinco River, tributary of Piracicaba River; NUP 5379, 2, 84.1–91.7 mm SL, São Paulo, Passa Cinco River, tributary of Piracicaba River; NUP 5722, 1, 90.5 mm SL, São Paulo, Passa Cinco River, tributary of Piracicaba River; NUP 6411, 17, 46.5–103.28 mm SL; NUP 6413, 13, 45.9–98.9 mm SL, São Paulo, Piracicaba River, tributary of Tietê River. Hypostomus pusarum: CAS 122221, 4, 94.4–141.7 mm SL, paratypes; CAS 122225, 1, 142.6 mm SL, holotype; NUP 4795, 11, 140.0–207.0 mm SL, Acauã River, Piranhas-Açu River basin; NUP 14683, 2, 103.1–135 mm SL, Piranhas, Piranhas-Açu River basin; NUP 14685, 10, 64.7–180.3 mm SL, Rio Grande do Norte, Ceará Mirim River basin; UFPB 7701, 25, 85.7–174.1 mm SL, Rio Grande do Norte, Jardim Angico; UFRN 1818, 6, 88.4–141.3 mm SL, Rio Grande do Norte, Apodi, Apodi River basin; UFPB 11566, 10, 122.6–172.8 mm SL, Poti River, Crateús, Ceará. Hypostomus regani: BMNH 1905.6.7.3, 174.2 mm SL, holotype, São Paulo, Piracicaba River. Hypostomus strigaticeps: BMNH 1907.7.6.1012, syntypes, 3, 75.7–160.0 mm SL, São Paulo, Piracicaba River, Tietê River basin; NUP 4017, 2, 72.8–100.0 mm SL, São Paulo, Ipuã, Ipanema River, Tietê River basin; NUP 4538, 11, 82.0–140 mm SL, São Paulo, Corumbataí River, tributary Tietê River. Hypostomus unae: MCP 41334, 3, 55.2–120.8 mm SL, Bahia, Panelinha River, tributary of Pardo River; MCP 41473, 10, 80.2–126.5 mm SL, Bahia, Preto do Costa River, tributary of de Contas River; NUP 9811, 5, 78.9–53.7 mm SL, Bahia, das Pedras River, de Contas River basin; NUP 9814, 81.5–102.7 mm SL, Bahia, Oricó River, de Contas River basin. Hypostomus vaillanti: UFRN 5648, 3, 167.5–240.2 mm SL; UFPB 11800, 4, 99.2–132.4 mm SL, Castelo do Piauí; UFPB 11801, 2, 102.5–112.7 mm SL, Poti River. Hypostomus yaku: NUP 15348, 6, 29.8–58.1 mm SL, Goiás, Quente River, Paranaíba river basin. Hypostomus sp. 3: UFPB 11117, 113.7 mm SL, Guaribas River, Vila Torrões, Picos, Piauí.
ACKNOWLEDGEMENTS
The authors are grateful to Elton França, Guilherme Moro, Luciano Barros-Neto, Lucas Medeiros, Matheus Lira, Márcio Silva, Yuri Carvalho-Rocha, Patrícia Charvet, Roney Paiva, Robson Ramos, Stefane Ramos, Uedson Jacobina, Leonardo Calado and Willian Severi, for assisting with field work and to researchers of the Laboratório de Sistemática e Morfologia de Peixes of Universidade Federal da Paraíba (LASEP/UFPB) for technical support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Finance Code 001, and CNPq Edital Universal 14/2013 (# 483878/2013–8). SMQL thanks the Conselho Nacional de Desenvolvimento Científico e Teconológico (CNPq) for the productivity grant (313644/2018–7).
REFERENCES
- Anjos MS, Bitencourt JA, Nunes LA, Sarmento-Soares LM, Carvalho DC, Armbruster JW, Affonso PR. Species delimitation based on integrative approach suggests reallocation of genus in Hypostomini catfish (Siluriformes, Loricariidae). Hydrobiologia. 2020; 847(2):563–78. https://link.springer.com/article/10.1007/s10750-019-04121-z
» https://link.springer.com/article/10.1007/s10750-019-04121-z - Barbosa JM, Soares EC, Cintra IHA, Hermann M, Araújo ARR. Perfil da ictiofauna da bacia do rio São Francisco/Profile of the fish fauna of the São Francisco river basin. Acta Fish Aquat Res. 2017; 5(1):70–90. https://seer.ufs.br/index.php/ActaFish/article/view/5862
» https://seer.ufs.br/index.php/ActaFish/article/view/5862 - Beck MW. ggord: Ordination Plots with ggplot2. R package version 0.11, 2017. https://zenodo.org/badge/latestdoi/35334615.
» https://zenodo.org/badge/latestdoi/35334615. - Berbel-Filho WM, Ramos TPA, Jacobina UP, Maia DJG, Torres RA, Lima SMQ. Updated checklist and DNA barcode-based species delimitations reveal taxonomic uncertainties among freshwater fishes from the mid-north-eastern Caatinga ecoregion, North-Eastern Brazil. J Fish Biol. 2018; 93(2):311–23. https://doi.org/10.1111/jfb.13758
» https://doi.org/10.1111/jfb.13758 - Boeseman M. The genus Hypostomus Lacépède, 1803, and its Surinam representatives (Siluriformes, Loricariidae). Zoologische Verhandelingen. 1968; 99:1–89.
- Bruford MW, Hanotte O, Brookfield JFY, Burke TA. Multilocus and single-locus DNA fingerprinting. In: Hoelzel CAR. Editor. Molecular Genetic Analysis of Populations. Oxford University Press. 1992; p.225–69.
- Cardoso YP, Almiron A, Casciotta J, Aichino D, Lizarralde MS, Montoya-Burgos JI. Origin of species diversity in the catfish genus Hypostomus (Siluriformes: Loricariidae) inhabiting the Paraná River basin, with the description of a new species. Zootaxa. 2012; 3453(1):69–83. https://doi.org/10.11646/zootaxa.3453.1.5
» https://doi.org/10.11646/zootaxa.3453.1.5 - Cardoso YP, Brancolini F, Protogino L, Paracampo A, Bogan S, Posadas P, Montoya-Burgos JI. An integrated approach clarifies the cryptic diversity in Hypostomus Lacépède 1803 from the Lower La Plata Basin. An Acad Bras Cienc. 2019; 91(2). https://doi.org/10.1590/0001-3765201920180131
» https://doi.org/10.1590/0001-3765201920180131 - Carvalho PH. Análises filogenéticas e filogeográficas do complexo de espécies Hypostomus ancistroides (Siluriformes: Loricariidae). [Doctoral thesis]. São Paulo: Universidade de São Paulo; 2011.
- Casatti L, Rocha FC, Pereira DC. Habitat use by two species of Hypostomus (Pisces, Loricariidae) in southeastern Brazilian streams. Biota Neotrop. 2005; 5(2):157–65.
- Costa WJEM, Amorim PF, Mattos JLO. Synchronic historical patterns of species diversification in seasonal aplocheiloid killifishes of the semi-arid Brazilian Caatinga. PloS ONE. 2018; 13(2). https://doi.org/10.1371/journal.pone.0193021
» https://doi.org/10.1371/journal.pone.0193021 - Dias AC, Zawadzki CH. Identification key and pictures of the Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) from the rio Ivaí, upper rio Paraná basin.Check List. 2018; 14(2):393–414. https://doi.org/10.15560/14.2.393
» https://doi.org/10.15560/14.2.393 - Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012; 29:1969–73. https://doi.org/10.1093/molbev/mss075
» https://doi.org/10.1093/molbev/mss075 - Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2021 http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.as
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.as - Fujisawa T, Barraclough TG. Delimiting species using single-locus data and the generalized mixed yule coalescent approach: A revised method and evaluation on simulated data sets. Syst Biol. 2013; 62(5):707–24. https://doi.org/10.1093/sysbio/syt033
» https://doi.org/10.1093/sysbio/syt033 - International Union for Conservation of Nature (IUCN). Standards and petitions committee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Prepared by the Standards and Petitions Committee; 2019. https://www.iucnredlist.org/resources/redlistguidelines
» https://www.iucnredlist.org/resources/redlistguidelines - Jolliffe IT, Uddin M, Vines SK. Simplied EOFs-Three alternatives to rotation. Clim Res. 2002; 20:271–79. https://www.int-res.com/articles/cr2002/20/c020p271.pdf
» https://www.int-res.com/articles/cr2002/20/c020p271.pdf - Knaack J. New Ancistrus species from the Rio Cuiba system, Brazil (Pisces, Siluriformes, Loricariidae). TFH Magazine. 1999; 47:150–55.
- Lima SMQ, Ramos TPA, Silva MJ, Rosa RS. Diversity, distribution, and conservation of the caatinga fishes: advances and challenges. In: Silva JMC, Leal IR, Tabarelli M., editors. Caatinga. Springer; 2017. p.97–131.
- Lleonart J, Salat J, Torres GJ. Removing allometric effects of body size in morphological analysis. J Theor Biol. 2000; 205(1):85–93. https://doi.org/10.1006/jtbi.2000.2043
» https://doi.org/10.1006/jtbi.2000.2043 - Lucena CAS, Calegari BB, Pereira EHL, Dallegrave E. O uso de óleo de cravo na eutanásia de peixes. Bol Soc Bras Ictiologia. 2013; 105:20–24.
- Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogeneti Evol. 2015; 82:269–88. https://doi.org/10.1016/j.ympev.2014.08.020
» https://doi.org/10.1016/j.ympev.2014.08.020 - Malabarba LR, Reis RE. Manual de técnicas para a preparação de coleções zoológicas.Soc Bras Zool. 1987; 36:1–14.
- Melo FAG, Buckup PA, Ramos TPA, do Nascimento Souza AK, Silva CMA, Costa TC, Torres AR. Fish fauna of the lower course of the Parnaíba River, northeastern Brazil. Bol Mus Biol Mello Leitão. 2016; (4):363–400.
- Mitteroecker P, Bookstein F. Linear discrimination, ordination, and the visualization of selection gradients in modern morphometrics. Evolutionary Biology. 2011; 38(1):100–14. https://doi.org/10.1007/s11692-011-9109-8
» https://doi.org/10.1007/s11692-011-9109-8 - Montoya-Burgos JI. Historical biogeography of the catfish genus Hypostomus (Siluriformes: Loricariidae), with implications on the diversification of Neotropical ichthyofauna. Mol Ecol. 2003; 12(7):1855–67. https://doi.org/10.1046/j.1365-294X.2003.01857.x
» https://doi.org/10.1046/j.1365-294X.2003.01857.x - De Oliveira Brandão K, Rocha-Reis DA, Garcia C, Pazza R, de Almeida-Toledo LF, Kavalco KF. Studies in two allopatric populations of Hypostomus affinis (Steindachner, 1877): the role of mapping the ribosomal genes to understand the chromosome evolution of the group. Comp Cytogenet. 2018; 12(1):1–12. https://doi.org/10.3897/CompCytogen.v12i1.22052
» https://doi.org/10.3897/CompCytogen.v12i1.22052 - Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. vegan: Community Ecology Package (R package version 2.5-6). 2019.
- Oyakawa OT, Akama A, Zanata AM. Review of the genus Hypostomus Lacépède, 1803 from rio Ribeira de Iguape basin, with description of a new species (Pisces, Siluriformes, Loricariidae). Zootaxa. 2005; 921:1–27.
- Penido IS, Pessali TC, Zawadzki CH. When destruction comes first: Two new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from a deeply-impacted river in the Rio São Francisco basin in Brazil. J Fish Biol. 2021; 98(5):1371–84. https://doi.org/10.1111/jfb.14674
» https://doi.org/10.1111/jfb.14674 - Pereira LH, Hanner R, Foresti F, Oliveira C. Can DNA barcoding accurately discriminate megadiverse Neotropical freshwater fish fauna? BMC Genet. 2013; 14(20). https://doi.org/10.1186/1471-2156-14-20
» https://doi.org/10.1186/1471-2156-14-20 - Pereira LH, Maia GM, Hanner R, Foresti F, Oliveira C. DNA barcodes discriminate freshwater fishes from the Paraíba do Sul River Basin, São Paulo, Brazil. Mitochondrial Dna. 2011; 22:71–79. https://doi.org/10.3109/19401736.2010.532213
» https://doi.org/10.3109/19401736.2010.532213 - De Queiroz LJ, Cardoso Y, Jacot-des-Combes C, Bahechar IA, Lucena CA, Py-Daniel LR, Torrente-Vilara G. Evolutionary units delimitation and continental multilocus phylogeny of the hyperdiverse catfish genus Hypostomus.Mol Phylogenet Evol. 2020; 145:106711. https://doi.org/10.1016/j.ympev.2019.106711
» https://doi.org/10.1016/j.ympev.2019.106711 - R Development Core Team. R: The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. https://www.r-project.org/
» https://www.r-project.org/ - Ramos TPA, Ramos RTC, Ramos SAQA. Ichthyofauna of the Parnaíba river Basin, Northeastern Brazil. Biota Neotrop. 2014; 14(1). https://doi.org/10.1590/S1676-06020140039
» https://doi.org/10.1590/S1676-06020140039 - Ramos TPA, Zawadzki CH, Ramos RTDC, Britski HA. Redescription of Hypostomus johnii, a senior synonym of Hypostomus eptingi (Siluriformes: Loricariidae), Northeastern Brazil. Neotrop Ichthyol. 2017; 15(2):e160064. https://doi.org/10.1590/1982-0224-20160064
» https://doi.org/10.1590/1982-0224-20160064 - Reis RE, Kullander SO, Ferraris Jr, C. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs. 2003.
- Rosa RS, Menezes NA, Britski HA, Costa WJE, Groth F. Diversidade, padrões de distribuição e conservação dos peixes da caatinga; In: Leal IR, Tabarelli M, Silva JMC, editors. Ecologia e Conservação da Caatinga. Recife: Editora Universitária da Universidade Federal de Pernambuco. 2003; p.135–80.
- Sá-Oliveira JC, Isaac VJ. Diet breadth and niche overlap between Hypostomus plecostomus (Linnaeus, 1758) and Hypostomus emarginatus (Valenciennes, 1840) (Siluriformes) from the coaracy nunes hydroelectric reservoir in Ferreira Gomes, Amapá-Brazil. Biota Amaz. 2015; 3(2). http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v3n2p116-125
» http://dx.doi.org/10.18561/2179-5746/biotaamazonia.v3n2p116-125 - Schaefer SA. Osteology of Hypostomus plecostomus (Linnaeus), with a phylogenetic analysis of the loricariid subfamilies (Pisces: Siluroidei). Contrib Sci. 1987; 394:1–31.
- Schlager S. Morpho and Rvcg - shape analysis in R: R-packages for geometric morphometrics, shape analysis and surface manipulations. In: Zheng G, Li S, Székely G, editors. Statistical shape and deformation analysis: methods, implementation and applications. Academic Press; 2017. p.217–56.
- Silva GSC, Roxo FF, Lujan NK, Tagliacollo VA, Zawadzki CH, Oliveira C. Transcontinental dispersal, ecological opportunity and origins of an adaptive radiation in the Neotropical catfish genus Hypostomus (Siluriformes: Loricariidae). Mol Ecol. 2016; 25(7):1511–29. https://doi.org/10.1111/mec.13583
» https://doi.org/10.1111/mec.13583 - Silva MJ, Costa BG, Ramos TPA, Auricchio P, Lima SMQ. Ichthyofauna of the Gurgueia river, Parnaíba river basin, northeastern Brazil. Check List. 2015; 11(5):1–8. https://doi.org/10.15560/11.5.1765
» https://doi.org/10.15560/11.5.1765 - Starks EC. The fishes of the Stanford Expedition to Brazil. Stanford, Leland Stanford Junior University Publications. 1913. 77p.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: molecular evolutionary genetics analysis, version 6.0. Mol Biol Evol. 2013; 30(12):2725–29. https://doi.org/10.1093/molbev/mst197
» https://doi.org/10.1093/molbev/mst197 - Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22:4673–80. https://doi.org/10.1093/nar/22.22.4673
» https://doi.org/10.1093/nar/22.22.4673 - Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD. DNA barcoding Australia’s fish species. Phil Trans R Soc B. 2005; 360(1462):1847–57. https://doi.org/10.1098/rstb.2005.1716
» https://doi.org/10.1098/rstb.2005.1716 - Weber C. The Hypostominae. In: Reis RE, Kullander SO, Ferraris Jr. CJ, editors. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucrs. 2003; p.351–72.
- Weber C Hypostomus dlouhyi, nouvelle espèce de poissonchat cuirassé du Paraguay (Pisces, Siluriformes, Loricariidae). Rev Suisse Zool. 1985; 92:955–68.
- Zanata AM, Pitanga BR. A new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from rio Itapicuru basin, Bahia State, Brazil. Zootaxa. 2016; 4137(2):223–32. https://doi.org/10.11646/zootaxa.4137.2.4
» https://doi.org/10.11646/zootaxa.4137.2.4 - Zawadzki CH, Birindelli JLO, Lima FCT. A new armored catfish species of the genus Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the upper rio Xingu basin, Brazil. Neotrop Ichthyol. 2012; 10(2):245–53. https://doi.org/10.1590/S1679-62252012000200003
» https://doi.org/10.1590/S1679-62252012000200003 - Zawadzki CH, Oliveira RRD, Debona T. A new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from the rio Tocantins-Araguaia basin, Brazil. Neotrop Ichthyol. 2013; 11(1):73–80. https://doi.org/10.1590/S1679-62252013000100008
» https://doi.org/10.1590/S1679-62252013000100008 - Zawadzki CH, Ramos TPA, Sabaj M Hypostomus sertanejo (Siluriformes: Loricariidae), new armoured catfish species from north-eastern Brazil. J Fish Biol. 2017; 91(1):317–30. https://doi.org/10.1111/jfb.13349
» https://doi.org/10.1111/jfb.13349 - Zawadzki CH, Renildo RDO, Oliveira ADS, Py-Daniel LR. Redescription of Hypostomus carinatus (Steindachner 1881) (Siluriformes: Loricariidae) from the rio Amazonas basin in Brazil. Zootaxa. 2020; 4750(2):191–203. https://doi.org/10.11646/zootaxa.4750.2.3
» https://doi.org/10.11646/zootaxa.4750.2.3 - Zhang J, Kapli P, Pavlidis P, Stamatakis A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics. 2013; 29(22):2869–76. https://doi.org/10.1093/bioinformatics/btt499
» https://doi.org/10.1093/bioinformatics/btt499 - Zuur A, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010; 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
» https://doi.org/10.1111/j.2041-210X.2009.00001.x
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Lustosa-Costa SY, Ramos TPA, Zawadzki CH, Lima SMQ. Review of the armoured catfish genus Hypostomus (Siluriformes: Loricariidae) from the Parnaíba River basin, Northeastern Brazil, with description of a new species. Neotrop Ichthyol. 2022; 20(1):e210126. https://doi.org/10.1590/1982-0224-2021-0126
Edited-by
Publication Dates
-
Publication in this collection
08 Apr 2022 -
Date of issue
2022
History
-
Received
25 Aug 2021 -
Accepted
18 Feb 2022